As it is not proper today to cure the eyes without the head nor the head without the body, so neither is it proper to cure the body without the mind, and this is the reason why so many diseases escape Greek physicians who are ignorant of the whole.
– Socrates
Read time: 15 minutes
Key Points:
- The GI tract has it own nervous system that cross-talks with the brain
- Our stress response and even emotions are regulated by our guts. This is the basis for modern neuro-gastroenterology and the biology of stress
- Modulating our microbiomes may be the target for future therapies
- Humans are not mice. Conclusions about stress induced gut dysfunction have to be contextualized.
- Don’t go to an island to see genetically modified, resurrected dinosaurs. Ever!
Coming Soon:
Part 2: Stress in our daily lives and the Development of Intestinal discomfort
Part 3: Strategies to combat stress and improve gut health
Stress sucks doesn’t it?
It seems to be everywhere and affecting everyone (much like contouring videos on Youtube). What’s worse, it’s not just in your head but also in your gut. The classic case of this is stage fright. Right before showtime, our stomachs go up and down like a roller coaster, we become nauseated, and it seems like our insides our quivering.
In fact, our language has supported this concept for a while. We talk about gut feelings, gut sense, feeling it in the gut, gut wrenching experience, butterflies in the stomach, going with your gut, a crisis giving us punch in the gut, or that the way to a (man’s) heart is through his stomach.
Emotion, intuition, food, and health are all linked together. The nexus of all of it is the GI tract.
What is stress anyways?
Stress can be divided into two elements – the stressor (i.e. the trigger) and the stress response. These can be different for different people at different times in their lives. For the purposes of this essay, the term “stress” will be used to refer to both.
It is used to be that the primary stressor in our lives was to avoid getting eaten, much like the poor lawyer in the original (and best) Jurassic Park film.
Acute stress can activate the fight or flight response, which triggers our adrenal glands to secrete norepinephrine. Our blood pressure and heart rate increase, our eyes dilate and blood shunts away from our GI tracts to our skeletal muscles. The need to defecate can occur in order to shed dead weight so we can get the $%^ out of there! Hence, Gennaro rushes to the restroom when the T-rex first cuts loose.
In modern times, chronic stress is a more prevalent problem. We have to drop kids off to soccer practice, pass midterms, pay rent, or rush to make a healthy dinner every night. Emotions like anxiety can add up over time.
End the Mind-Body Dualism
Renee Descartes’s (“I think therefore I am”) created the widest gap in human biology: that between mind and body. For hundreds of years since, that philosophical premise has undergirded much thinking in Western Civilization and medicine. Modern gastroenterology is proving him wrong. We are not just talking heads on sticks. The brain and the body are actually intimately linked together. There is an impressive array of cross-talk between our guts and brains. They work in concert together in fascinating ways we are only beginning to uncover through modern scientific tools.
For example, the number of nerves in our gut is almost as many as in our brain. Many of neurotransmitters, including most of the serotonin (colloquially known as the “good mood” chemical) in the brain, are actually made in the intestines in so-called enterochromaffin cells (ECF). In fact, scientists now regularly speak of the enteric (or intestinal) nervous system to complement the traditional ideas of the central (brain and spinal cord) and peripheral (body) nervous system.
From mice (they’ll come up again later) scientists increasingly appreciate the notion that commensal gut microbes help shape brain development and the programming of the Hypothalamus-Pituitary-Adrenal Axis (HPA), the central pathway of the stress response. Yes, this is the same response that compelled our friendly lawyer to try to escape the T-Rex and empty himself in the process.
A Closer Look at Stress in the Gut
A detailed explanation of the bacterial ecosystem of our guts will be saved for later. For now, understand that commonly cited estimates of 10 times the number of bacteria as human cells in a person are likely inaccurate. Rosner provides a number between 30 and 400 × 1012 bacterial cells in people with an average 2,000,000 microbial genes (100 x that of the number of human genes). Gut bacteria are involved in a variety of essential functions of the intestines: nutrient and drug metabolism, mucosal defense of gut barrier, and immunomodulation.
A decade’s worth of animal studies have shown that stress can reduce microbial diversity in the gut and that these changes themselves can propagate neurochemical changes in the brain. In particular, decreases in lactobacilli have prompted changes in the serotonin pathway, which can impair mood and sleep and enhance sensations of pain.
Four types of studies have helped shape the ideas around the new microbiome: antibiotic studies, probiotic studies, germ-free studies, and infection studies. In all of these cases, modulation of the internal bacterial ecosystem of the guts of mice and in some cases, people, has yielded dramatic new insights into how our insides work.
Stress can alter gut function in a variety of ways.
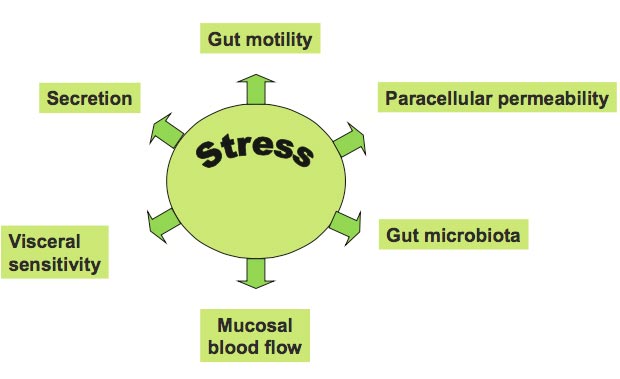
Animals raised in a germ-free environment show exaggerated responses in this HPA axis, the primary stress mechanism of the body. This is an effect that can be partially reversed by probiotics including those with strains of Bifidobacterium infantis.
Stress can also loosen the paracellular junctions between epithelial cells of the gut, allowing translocation of bacteria and causing local immunological activation. This has been colloquially referred to as “leaky gut.” In fact, one recent study in people demonstrated that small bowel intestinal permeability increased, as did salivary cortisol in response to acute stress. What was that stressor? You guessed it – public speaking.
photo cred: edx.org
The clinical consequence of these mild changes in urinary mannitol is unclear, but is one piece of a very large jigsaw puzzle that involves the intersection of neurochemistry, immunology, endocrinology, psychology, gastroenterology, and the modern microbiome.
Tantalizing Clues
The link between the endocrine, behavioral and immune systems in response to stress is centered on a family of powerful neuropeptides called (CRF, urocortin 1, urocortin 2 and urocortin 3). CRF can modulate gut via enhanced inflammation, increased intestinal permeability, and contribution to visceral hypersensitivity, and altered motility. In other words, one of the primary regulators of stress hormones in the body directly affects the major features and functions of the intestines themselves.
In fact, the gut microbiota can be considered an endocrine organ of sorts.
Gut microbiota may modulate the sensation of pain and some probiotics may inhibit this hypersensitivity. A small pilot study of healthy women at UCLA demonstrated that ingestion of yogurt modulated response to an emotional recognition task and altered activity in parts of the prefrontal cortex on fMRI (functional magnetic resonance imaging). In essence, signals from the intestine in response to food can alter the brain and perhaps even mood. We also know that short chain fatty acids (SCFA), which are byproducts of bacterial metabolism of certain carbohydrates, can maintain the integrity of the blood-brain barrier. These lines of thinking bring new light to the phrase you are what you eat.
Put together, some researchers have hypothesized the existence of a brain-gut-microbiota axis that tilts on a fulcrum of stress.
A postulated mechanism of the brain- gut-microbiota axis.
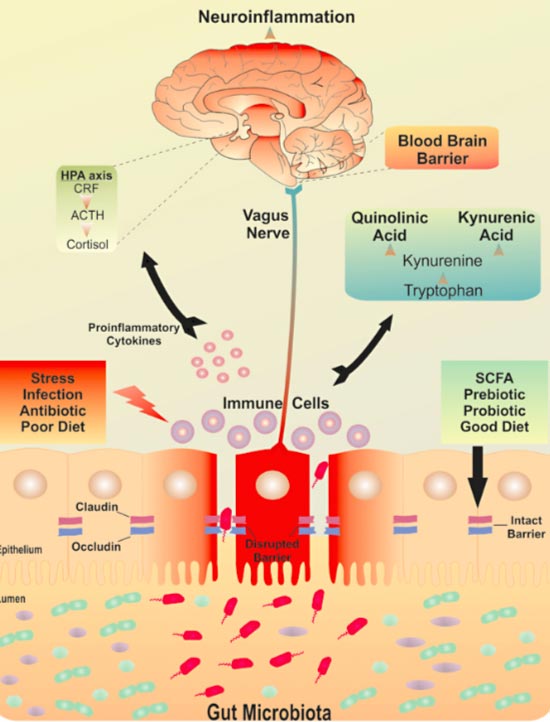
“I gut a feeling ooh ooh that tonight’s gonna be a good night.” Yes, that is a Black Eye Peas pun.
(Yes I know Fergie is out)
Exposure to chronic stress may actual remodel parts of the brain itself in a deleterious example of so-called neuroplasticity. In other words, all of these changes are often self-reinforcing. Chronic stress can prompt neurological changes in the functioning of the guts of susceptible people. These changes can, in turn, create more stress and anxiety. So when people tell patients with IBS, “it’s all in your head” that is partially true. But, it is also in your gut and that is valuable to know.
What We DON’T Know about Stress and the Gut
“In general, the problem of causality in microbiome studies is substantial,” says Rob Knight, a microbiologist at the University of California, San Diego. “It’s very difficult to tell if microbial differences you see associated with diseases are causes or consequences.” Dr. Knight is one of the leading researchers in this field, so his comments bear weight.
Conjecture trumps clear answers at this point. Most research in the area of the microbiome, stress, and intestinal health is descriptive in nature. By that I mean scientists characterize various phenomena they observe in germ-free mice and other animals. What we need to know are mechanisms that underlie these findings and then use that to do predictive work. Can we determine which people under which stressors will develop what particular intestinal problems and when? That is the holy grail of modern neuro-gastroenterology. We are very far away from answering this question.
How do probiotics work? We don’t know
How many good and bad bacteria do we have? We don’t know.
Why do bacteria influence our brain’s limbic system? We don’t know.
Does a particular diet allow me to manipulate my microbiome to feel better? We don’t know.
If I relax more and clean up my diet, will that fix my gut problems? We don’t know (but that may be a good place to start).
What’s the best to create genetically modified dinosaurs? We really don’t know.
All of this should give you pause when seeing advertisements for probiotics, digestive enzymes or other natural “gut health” wonder drugs. The wondrous ecosystem of insides is too complex to be fixed with “8 flat belly foods.”
In part two we will look at how stress influences our guts on a day to day basis…
References:
[1] Kelly JR et al Breaking Down the Barriers: The Gut Microbiome, Intestinal Permeability and Stress-related Psychiatric Disorders Frontiers in Cellular Neuroscience 9:392 · November 2015
[2] Stress & the gut-brain axis: Regulation by the microbiome. Neurobiol Stress. 2017 Dec; 7: 124–136.
[3] Still from Jurassic Park. Universal Studios. Steven Spielberg don’t sue me.
[4] James Corden. The Late Late Show, CBS.
[5] Konturek, P. C., Brzozowski, T., & Konturek, S. J. (2011). Stress and the gut: pathophysiology, clinical consequences, diagnostic approach and treatment options. Journal of physiology and pharmacology : an official journal of the Polish Physiological Society, 6, 591–599
[6] Bravo et al Communication between gastrointestinal bacteria and the nervous system. Current Opinion in Pharmacology. Volume 12, Issue 6, December 2012, Pages 667-672
[7] Social Stress Leads to Changes in Gut Bacteria, Georgia State Study Finds
https://news.gsu.edu/2018/03/08/social-stress-leads-changes-gut-bacteria-study-finds
[8] Peter Smith. The Tantalizing links between gut microbes and the brain. Nature News Feature. October 14 2015.
[9] Charles Schmidt. Thinking from the Gut. Nature. February 2015.
[10] Chang YM et al. Does stress induce bowel dysfunction? Expert Rev Gastroenterol Hepatol. 2014 Aug; 8(6): 583–585. 2014 May 31. doi: 10.1586/17474124.2014.911659
[11] Dinan TG, Cryan JF Regulation of the stress response by the gut microbiota: implications for psychoneuroendocrinology. Psychoneuroendocrinology. 2012 Sep;37(9):1369-78. doi: 10.1016/j.psyneuen.2012.03.007.
[12] Verdu EF Probiotics effects on gastrointestinal function: beyond the gut? Neurogastroenterol Motil. 2009 May;21(5):477-80. doi: 10.1111/j.1365-2982.2009.01297.x.
[13]Bhatia V and Tandon RK. Stress a nd the gastrointestinal tract. J Gastroenterol Hepatol. 2005 Mar;20(3):332-9.
[14] Vanuytsel T et al. Psychological stress and corticotropin-releasing hormone increase intestinal permeability in humans by a mast cell-dependent mechanism. Gut. 2014 Aug;63(8):1293-9. doi: 10.1136/gutjnl-2013-305690. Epub 2013 Oct 23.
[15] Lipowski et al “Psychosomatic Medicine: Past and Present. Part 1.” Historical Background. Can J Psychiatry 1986;31:2-7.
[16] Rosner, J.L. Ten times more microbial cells than body cells in humans? Microbe 9, 47 (2014).
[17] http://www.rap-up.com/2017/06/01/fergie-leaves-the-black-eyed-peas/
[18] Sherwin E et. al. “A Gut (Microbiome) Feeling about the Brain.” Curr Opin Gastroenterol. 2016 Mar;32(2):96-102.
Lipowski ZJ. Psychosomatic medicine: Past and present.
Part 1: Historical background. Can. J. Psychiatry 1986;
31: 2–7.
[19] Jandhyala et al Role of the normal gut microbiota World J Gastroenterol. 2015 Aug 7; 21(29): 8787–8803.